close
Choose Your Site
Global
Social Media
| Availability: | |
|---|---|
| Quantity: | |








7789-24-4
7789-24-4
Lithium fluoride(LiF)
CAS 7789-24-4
Alias:High purity lithium fluoride, PURATRONIC|R (METALS BASIS), lithium chloride monohydrate, LiF
Molecular formula:FLi
Molecular weight:25.94
Structural formula:
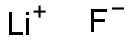
1.Performance and use:
Lithium fluoride is white powder with sodium chloride type crystal structure. Relative density 2.640, melting point 848℃, boiling point 1673℃. In 1100 ~ 1200 ℃ began to volatilise, the vapour is Chemicalbook alkaline. Lithium fluoride is slightly soluble in water, insoluble in alcohol and other organic solvents. At room temperature, lithium fluoride is soluble in nitric acid and sulfuric acid, but insoluble in hydrochloric acid, can be generated with hydrofluoric acid Li2HF acid salt.
Lithium fluoride can be widely used as a co-solvent in glass-lining, copper, aluminium welding process and salt melting process; also recommended as a heat-carrying agent for the storage of solar radiation heat energy in aerospace technology Chemicalbook; can also be used in the electrolysis of aluminium and metallurgical industry. High-purity lithium fluoride is used in the manufacture of fluoride glass, and is also used to make prisms for spectrometers and X-ray monochromators. Raw material for lithium-ion batteries.
Used as desiccant and flux, also used in the manufacture of optical glass.
Used as additive for aluminium electrolysis and rare earth electrolysis, optical glass manufacturing, desiccant, flux, etc.
Optoelectronic material research for insulating material coating, electrode modification layer.
Battery-grade lithium fluoride is one of the key raw materials for the production of lithium hexafluorophosphate, a common electrolyte for lithium-ion batteries.
Used in the manufacture of spectrometers and X-ray monochromator prisms, used as a carrier for the proliferation reaction.
2.Technical indicators:
| Item | Specification(%) | Result(%) |
| LiF | ≥99.95 | 99.95min |
| K | ≤0.001 | 0.00086 |
| Na | ≤0.001 | 0.00074 |
| Ca | ≤0.001 | 0.0001 |
| Mg | ≤0.001 | 0.0001 |
| Fe | ≤0.0005 | 0.0002 |
| Al | ≤0.001 | <0.0001 |
| Zn | ≤0.0005 | <0.0001 |
| Ni | ≤0.0005 | <0.0001 |
| Pb | ≤0.0005 | <0.0001 |
| Cu | ≤0.0005 | <0.0001 |
| Mn | ≤0.0005 | <0.0001 |
| Cl | ≤0.005 | 0.0002 |
| SO42 | ≤0.005 | 0.0002 |
| SiO2 | ≤0.015 | 0.0002 |
| H2O | ≤0.01 | 0.0086 |
3.Usage
There are two methods: the neutralisation method and the complex decomposition method. The neutralisation method is mostly used in industrial production. Neutralisation method is to prepare lithium fluoride by reacting lithium carbonate or lithium hydroxide with hydrofluoric acid.Li2COChemicalbook3+2HF=2LiF+CO2+H2OAdd solid lithium carbonate into the hydrogen fluoride solution to precipitate LiF crystals in the reaction, and then filter and dry the product.
4.Packaging and storage
25kg/bag
Related keywords:
Lithium fluoride
CAS 7789-24-4
Industrial Grade LiF
Industrial Grade LiF
Industrial Grade LiF
LiF
Lithium fluoride(LiF)
CAS 7789-24-4
Alias:High purity lithium fluoride, PURATRONIC|R (METALS BASIS), lithium chloride monohydrate, LiF
Molecular formula:FLi
Molecular weight:25.94
Structural formula:
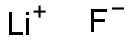
1.Performance and use:
Lithium fluoride is white powder with sodium chloride type crystal structure. Relative density 2.640, melting point 848℃, boiling point 1673℃. In 1100 ~ 1200 ℃ began to volatilise, the vapour is Chemicalbook alkaline. Lithium fluoride is slightly soluble in water, insoluble in alcohol and other organic solvents. At room temperature, lithium fluoride is soluble in nitric acid and sulfuric acid, but insoluble in hydrochloric acid, can be generated with hydrofluoric acid Li2HF acid salt.
Lithium fluoride can be widely used as a co-solvent in glass-lining, copper, aluminium welding process and salt melting process; also recommended as a heat-carrying agent for the storage of solar radiation heat energy in aerospace technology Chemicalbook; can also be used in the electrolysis of aluminium and metallurgical industry. High-purity lithium fluoride is used in the manufacture of fluoride glass, and is also used to make prisms for spectrometers and X-ray monochromators. Raw material for lithium-ion batteries.
Used as desiccant and flux, also used in the manufacture of optical glass.
Used as additive for aluminium electrolysis and rare earth electrolysis, optical glass manufacturing, desiccant, flux, etc.
Optoelectronic material research for insulating material coating, electrode modification layer.
Battery-grade lithium fluoride is one of the key raw materials for the production of lithium hexafluorophosphate, a common electrolyte for lithium-ion batteries.
Used in the manufacture of spectrometers and X-ray monochromator prisms, used as a carrier for the proliferation reaction.
2.Technical indicators:
| Item | Specification(%) | Result(%) |
| LiF | ≥99.95 | 99.95min |
| K | ≤0.001 | 0.00086 |
| Na | ≤0.001 | 0.00074 |
| Ca | ≤0.001 | 0.0001 |
| Mg | ≤0.001 | 0.0001 |
| Fe | ≤0.0005 | 0.0002 |
| Al | ≤0.001 | <0.0001 |
| Zn | ≤0.0005 | <0.0001 |
| Ni | ≤0.0005 | <0.0001 |
| Pb | ≤0.0005 | <0.0001 |
| Cu | ≤0.0005 | <0.0001 |
| Mn | ≤0.0005 | <0.0001 |
| Cl | ≤0.005 | 0.0002 |
| SO42 | ≤0.005 | 0.0002 |
| SiO2 | ≤0.015 | 0.0002 |
| H2O | ≤0.01 | 0.0086 |
3.Usage
There are two methods: the neutralisation method and the complex decomposition method. The neutralisation method is mostly used in industrial production. Neutralisation method is to prepare lithium fluoride by reacting lithium carbonate or lithium hydroxide with hydrofluoric acid.Li2COChemicalbook3+2HF=2LiF+CO2+H2OAdd solid lithium carbonate into the hydrogen fluoride solution to precipitate LiF crystals in the reaction, and then filter and dry the product.
4.Packaging and storage
25kg/bag
Related keywords:
Lithium fluoride
CAS 7789-24-4
Industrial Grade LiF
Industrial Grade LiF
Industrial Grade LiF
LiF
content is empty!