close
Choose Your Site
Global
Social Media
| Availability: | |
|---|---|
| Quantity: | |








12057-24-8
bosschemical
12057-24-8
Lithium oxide
CAS No. 12057-24-8
alias:dilithiumoxide;Li2O;Lithium oxide (Li2O);lithiummonoxide;Lithiumoxid;lithiumoxide(li2o);Lithium Oxide,98.5%;oxydedelithium.
Molecular formula:Li2O
Molecular weight:29.88
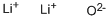
1. Performance and use:
Lithium oxide (Li2O) is one of simplest ionic oxides and it is isoelectronic to H2O. Two lithium atoms will each give one electron to the oxygen atom. forms the ionic bond between lithium and oxygen. The formula for lithium oxide is Li2O.
Lithium oxide is very corrosive. It reacts with water to make lithium hydroxide. It is toxic because of its strong alkalinity (being a base).
Lithium oxide is a highly insoluble thermally stable Lithium source suitable for glass, optic and ceramic applications. Lithium oxide is a white solid also known as lithia, it is produced when lithium metal burns in the presence of oxygen. Oxide compounds are not conductive to electricity. However, certain perovskite structured oxides are electronically conductive finding application in the cathode of solid oxide fuel cells and oxygen generation systems. They are compounds containing at least one oxygen anion and one metallic cation.
Lithium oxide is used as a flux in ceramic glazes; and creates blues with copper and pinks with cobalt. Lithium oxide reacts with water and steam, forming lithium hydroxide and should be isolated from them. Its usage is also being investigated for non-destructive emission spectroscopy evaluation and degradation monitoring within thermal barrier coating systems. It can be added as a co-dopant with yttria in the zirconia ceramic top coat, without a large decrease in expected service life of the coating.
Lithium oxide is used as a flux in ceramic glazes; and creates blues with copper and pinks with cobalt.
2. Technical indicators
| Items tested | Specifications(%) | Results (%) |
| Li2O | ≥98.5 | 98.97 |
| Li2CO3 | ≤2.5 | 1.12 |
| Na | ≤0.03 | 0.0025 |
| K | ≤0.03 | 0.0021 |
| Fe | ≤0.01 | 0.0015 |
| Ca | ≤0.01 | 0.0032 |
| Zn | ≤0.001 | 0.0005 |
| Mg | ≤0.001 | 0.0001 |
| Pb | ≤0.001 | 0.0001 |
| Cr | ≤0.001 | 0.0002 |
3. Packaging and storage
Lithium oxide is packaged in plastic woven bags lined with polyethylene bags, with a net weight of 25kg per bag, which can also be determined according to user needs. Store in a cool and ventilated place indoors, avoid moisture and exposure, and the storage period is twelve months.
Related keywords:
Lithium oxide
CAS 12057-24-8
Spectrally pure reagent
Glass ceramics field
Battery material
li2o lithium oxide
lithium cobalt oxide battery
lithium oxide powder
Lithium oxide
CAS No. 12057-24-8
alias:dilithiumoxide;Li2O;Lithium oxide (Li2O);lithiummonoxide;Lithiumoxid;lithiumoxide(li2o);Lithium Oxide,98.5%;oxydedelithium.
Molecular formula:Li2O
Molecular weight:29.88
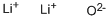
1. Performance and use:
Lithium oxide (Li2O) is one of simplest ionic oxides and it is isoelectronic to H2O. Two lithium atoms will each give one electron to the oxygen atom. forms the ionic bond between lithium and oxygen. The formula for lithium oxide is Li2O.
Lithium oxide is very corrosive. It reacts with water to make lithium hydroxide. It is toxic because of its strong alkalinity (being a base).
Lithium oxide is a highly insoluble thermally stable Lithium source suitable for glass, optic and ceramic applications. Lithium oxide is a white solid also known as lithia, it is produced when lithium metal burns in the presence of oxygen. Oxide compounds are not conductive to electricity. However, certain perovskite structured oxides are electronically conductive finding application in the cathode of solid oxide fuel cells and oxygen generation systems. They are compounds containing at least one oxygen anion and one metallic cation.
Lithium oxide is used as a flux in ceramic glazes; and creates blues with copper and pinks with cobalt. Lithium oxide reacts with water and steam, forming lithium hydroxide and should be isolated from them. Its usage is also being investigated for non-destructive emission spectroscopy evaluation and degradation monitoring within thermal barrier coating systems. It can be added as a co-dopant with yttria in the zirconia ceramic top coat, without a large decrease in expected service life of the coating.
Lithium oxide is used as a flux in ceramic glazes; and creates blues with copper and pinks with cobalt.
2. Technical indicators
| Items tested | Specifications(%) | Results (%) |
| Li2O | ≥98.5 | 98.97 |
| Li2CO3 | ≤2.5 | 1.12 |
| Na | ≤0.03 | 0.0025 |
| K | ≤0.03 | 0.0021 |
| Fe | ≤0.01 | 0.0015 |
| Ca | ≤0.01 | 0.0032 |
| Zn | ≤0.001 | 0.0005 |
| Mg | ≤0.001 | 0.0001 |
| Pb | ≤0.001 | 0.0001 |
| Cr | ≤0.001 | 0.0002 |
3. Packaging and storage
Lithium oxide is packaged in plastic woven bags lined with polyethylene bags, with a net weight of 25kg per bag, which can also be determined according to user needs. Store in a cool and ventilated place indoors, avoid moisture and exposure, and the storage period is twelve months.
Related keywords:
Lithium oxide
CAS 12057-24-8
Spectrally pure reagent
Glass ceramics field
Battery material
li2o lithium oxide
lithium cobalt oxide battery
lithium oxide powder
content is empty!