close
Choose Your Site
Global
Social Media
| Availability: | |
|---|---|
| Quantity: | |








109-97-7
bosschemical
109-97-7
Pyrrole
CAS No.109-97-7
Alias:4-methyl(di-pyrrol-2-yl-methtyl)pyridinium iodide;PYRROLE;1-Aza-2,4-cyclopentadiene;Divinyleneimine;Monopyrrole;Parzate;Pyrrhol;Pyrrol
Molecular formula:C4H5N
Molecular weight:67.09
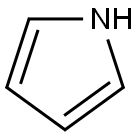
Structural formula:
1. Performance and use:
The five atoms on the pyrrole ring are all sp2 hybridised and are in the same plane. The pair of p orbitals occupied by the unshared electrons of the nitrogen atom are parallel and overlap with the p orbitals of the four carbon atoms to form a 5-atom, 6 π-electron, closed conjugated system, which is aromatic and susceptible to electrophilic substitution reactions. Therefore, the basicity of pyrrole nitrogen atom is small (pKb13.6); on the contrary, the hydrogen bound on the nitrogen atom, is weakly acidic. In addition, the pyrrole ring, like benzene and other aromatic compounds, can undergo nitration, sulphonation, diazo coupling reactions, and Friedel-Crafts type acylation reactions. This reaction can biologically 2-position substituted compounds. The nitrogen atom in the pyrrole molecule is sp2 hybridised, the unshared electron pair occupies the p-orbital, and the p-orbitals of the four sp2 hybridised carbon atoms overlap in parallel to form a six-electron conjugated system, which is aromatic, and can undergo electrophilic substitution reactions. The unshared electron pair of the nitrogen atom in the pyrrole molecule participates in the conjugation of the ring system, so it is very weakly combined with H+ and does not show basicity. Due to the relative reduction of electron density on the nitrogen atom, the hydrogen atom connected to the nitrogen atom can leave in the form of positive ions, thus pyrrole has weak acidity. Ionisation constant Ka=10-15, can react with solid potassium hydroxide to form a salt. Applications Many derivatives of pyrrole are important drugs and substances with strong physiological activity, such as chlorophyll and haemoglobin. Pyrrole is the basic structural unit of haemoglobin, chlorophyll, bile pigments, certain amino acids, several alkaloids and some enzymes, and these compounds have strong physiological activity and drug functions. Vitamin B12, stomach longing, sea people oxalic acid (roundworm medicine), lincomycin (antimicrobial) and other drugs contain hydrogenated pyrrole cyclic structure. 1979 years later, it was found that pyrrole can be electrochemical oxidation can be produced by flexible conductive polymer film, conductivity up to 104S / m, and has good stability.
2.Technical indicators:
Item | Test Result |
Appearance | Brown liquid |
| Purity | 85.4% |
Water | 3.78% |
3. Packaging and storage:
Packaging specification:25kg/drum, also can be determined according to user's need.
During transportation, it is necessary to protect against sunlight, rain, and high temperatures.
The product should be stored in a cool, dark, ventilated place, away from sources of fire.
4.Safety protection:
Pay attention to labor protection when handling it and avoid contact with skin, eyes, etc.After contact, rinse immediately with plenty of water
Related keywords:
Pyrrole
CAS No.109-97-7
Pyrrole
CAS No.109-97-7
Alias:4-methyl(di-pyrrol-2-yl-methtyl)pyridinium iodide;PYRROLE;1-Aza-2,4-cyclopentadiene;Divinyleneimine;Monopyrrole;Parzate;Pyrrhol;Pyrrol
Molecular formula:C4H5N
Molecular weight:67.09
Structural formula:

1. Performance and use:
The five atoms on the pyrrole ring are all sp2 hybridised and are in the same plane. The pair of p orbitals occupied by the unshared electrons of the nitrogen atom are parallel and overlap with the p orbitals of the four carbon atoms to form a 5-atom, 6 π-electron, closed conjugated system, which is aromatic and susceptible to electrophilic substitution reactions. Therefore, the basicity of pyrrole nitrogen atom is small (pKb13.6); on the contrary, the hydrogen bound on the nitrogen atom, is weakly acidic. In addition, the pyrrole ring, like benzene and other aromatic compounds, can undergo nitration, sulphonation, diazo coupling reactions, and Friedel-Crafts type acylation reactions. This reaction can biologically 2-position substituted compounds. The nitrogen atom in the pyrrole molecule is sp2 hybridised, the unshared electron pair occupies the p-orbital, and the p-orbitals of the four sp2 hybridised carbon atoms overlap in parallel to form a six-electron conjugated system, which is aromatic, and can undergo electrophilic substitution reactions. The unshared electron pair of the nitrogen atom in the pyrrole molecule participates in the conjugation of the ring system, so it is very weakly combined with H+ and does not show basicity. Due to the relative reduction of electron density on the nitrogen atom, the hydrogen atom connected to the nitrogen atom can leave in the form of positive ions, thus pyrrole has weak acidity. Ionisation constant Ka=10-15, can react with solid potassium hydroxide to form a salt. Applications Many derivatives of pyrrole are important drugs and substances with strong physiological activity, such as chlorophyll and haemoglobin. Pyrrole is the basic structural unit of haemoglobin, chlorophyll, bile pigments, certain amino acids, several alkaloids and some enzymes, and these compounds have strong physiological activity and drug functions. Vitamin B12, stomach longing, sea people oxalic acid (roundworm medicine), lincomycin (antimicrobial) and other drugs contain hydrogenated pyrrole cyclic structure. 1979 years later, it was found that pyrrole can be electrochemical oxidation can be produced by flexible conductive polymer film, conductivity up to 104S / m, and has good stability.
2.Technical indicators:
Item | Test Result |
Appearance | Brown liquid |
| Purity | 85.4% |
Water | 3.78% |
3. Packaging and storage:
Packaging specification:25kg/drum, also can be determined according to user's need.
During transportation, it is necessary to protect against sunlight, rain, and high temperatures.
The product should be stored in a cool, dark, ventilated place, away from sources of fire.
4.Safety protection:
Pay attention to labor protection when handling it and avoid contact with skin, eyes, etc.After contact, rinse immediately with plenty of water
Related keywords:
Pyrrole
CAS No.109-97-7
content is empty!