close
Choose Your Site
Global
Social Media
Calcium titanate, also known as calcium titanium trioxide, chemical formula CaTiO3, an inorganic substance. It has a yellow crystalline appearance and is insoluble in water. The first calcium titanium ore found in history, is the natural mineral calcium titanate (CaTiO3), which was discovered in 1839 by the German chemist Gustav Roth in the Ural Mountains of Russia during an expedition. Stable Chemicalbook at room temperature and pressure, high thermal decomposition discharges toxic calcium and titanium-containing fumes. Calcium titanate belongs to the cubic crystal system, titanium ions and six oxygen ions form octahedral coordination, the coordination number of 6; calcium ions are located in the cavity formed by the octahedron, the coordination number of 12. Many useful materials use this structure structure (such as barium titanate), or a deformation of the structure (such as yttrium-barium-copper oxide).
| Availability: | |
|---|---|
| Quantity: | |








12049-50-2
bosschemical
12049-50-2
CALCIUM TITANATE
CAS No.12049-50-2
Alias:CALCIUM TITANATE, 99.5%;Calciumtitaniumtrioxide;ct;Calcium titanium oxide, 99+% (metals basis);calcium metatitanate;Calciumtitanate,99%;titanate calcium;titanatitanium calcium oxide
Molecular formula:CaO3Ti
Molecular weight:135.94
Structural formula:
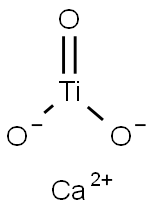
1. Performance and use:
Calcium titanate is a basic inorganic dielectric material with excellent dielectric, temperature, mechanical and optical properties, and is widely used in ceramic capacitors, PTC thermistors, microwave antennas, filters and stainless steel welding rods. Calcite is the name of the calcium titanate mineral, the structure of which is involved in many inorganic crystalline materials. An in-depth understanding of the structure of CaTiO and its variations will play a significant role in the research and development of inorganic functional materials. Calcium titanate (CaTChemicalbookiO3) is a cubic crystal system, and its structure can be regarded as the small Ti4+ is at the apex of the cubic cell, the large Ca2+ is at the centre of the cell, and O2- is at the centre of the prisms of the cell. Each Ti4+ is surrounded by 6 O2- coordinated in an ortho-octahedral shape, and each Ca2+ is surrounded by 12 O2- coordinated in a cubo-octahedral shape (type A). The cuboctahedron has 24 prongs, 12 vertices, and consists of 14 faces, of which 14 faces are six squares and eight square triangles.
Calcium titanate, also known as calcium titanium trioxide, chemical formula CaTiO3, an inorganic substance. It has a yellow crystalline appearance and is insoluble in water. The first calcium titanate discovered in history was the natural mineral calcium titanate (CaTiO3), which was discovered in 1839 by the German chemist Gustav Roth during an expedition to the Ural Mountains in Russia. Stable Chemicalbook at room temperature and pressure, high thermal decomposition discharges toxic calcium and titanium-containing fumes. Calcium titanate belongs to the cubic crystal system, titanium ions and six oxygen ions form octahedral coordination, the coordination number of 6; calcium ions are located in the cavity formed by the octahedron, the coordination number of 12. Many useful materials use this structure structure (such as barium titanate), or a deformation of the structure (such as yttrium-barium-copper oxide).
2.Technical indicators:
Type | CT-1 | CT-2 | CT-3 | CT-4 |
Purity (wt%) | ≥99.5 | ≥99 | ≥99 | Adjustable |
MgO (wt%) | <0.05 | <0.10 | <1.00 | <3.00 |
Fe2O3 (wt%) | <0.05 | <0.10 | <0.50 | <3.00 |
K2O+Na2O (wt%) | <0.05 | <0.10 | <0.50 | Pb<0.01 |
Al2O3 (wt%) | <0.10 | <0.20 | <0.50 | <1.00 |
SiO2 (wt%) | <0.10 | <0.20 | <0.50 | <3.00 |
3. Packaging and storage:
Packaging specification:25kg/bag, also can be determined according to user's need.
During transportation, it is necessary to protect against sunlight, rain, and high temperatures.
The product should be stored in a cool, dark, ventilated place, away from sources of fire.
4.Safety protection:
Pay attention to labor protection when handling it and avoid contact with skin, eyes, etc.After contact, rinse immediately with plenty of water
Related keywords:
CALCIUM TITANATE
CAS No.12049-50-2
CALCIUM TITANATE
CAS No.12049-50-2
Alias:CALCIUM TITANATE, 99.5%;Calciumtitaniumtrioxide;ct;Calcium titanium oxide, 99+% (metals basis);calcium metatitanate;Calciumtitanate,99%;titanate calcium;titanatitanium calcium oxide
Molecular formula:CaO3Ti
Molecular weight:135.94
Structural formula:

1. Performance and use:
Calcium titanate is a basic inorganic dielectric material with excellent dielectric, temperature, mechanical and optical properties, and is widely used in ceramic capacitors, PTC thermistors, microwave antennas, filters and stainless steel welding rods. Calcite is the name of the calcium titanate mineral, the structure of which is involved in many inorganic crystalline materials. An in-depth understanding of the structure of CaTiO and its variations will play a significant role in the research and development of inorganic functional materials. Calcium titanate (CaTChemicalbookiO3) is a cubic crystal system, and its structure can be regarded as the small Ti4+ is at the apex of the cubic cell, the large Ca2+ is at the centre of the cell, and O2- is at the centre of the prisms of the cell. Each Ti4+ is surrounded by 6 O2- coordinated in an ortho-octahedral shape, and each Ca2+ is surrounded by 12 O2- coordinated in a cubo-octahedral shape (type A). The cuboctahedron has 24 prongs, 12 vertices, and consists of 14 faces, of which 14 faces are six squares and eight square triangles.
Calcium titanate, also known as calcium titanium trioxide, chemical formula CaTiO3, an inorganic substance. It has a yellow crystalline appearance and is insoluble in water. The first calcium titanate discovered in history was the natural mineral calcium titanate (CaTiO3), which was discovered in 1839 by the German chemist Gustav Roth during an expedition to the Ural Mountains in Russia. Stable Chemicalbook at room temperature and pressure, high thermal decomposition discharges toxic calcium and titanium-containing fumes. Calcium titanate belongs to the cubic crystal system, titanium ions and six oxygen ions form octahedral coordination, the coordination number of 6; calcium ions are located in the cavity formed by the octahedron, the coordination number of 12. Many useful materials use this structure structure (such as barium titanate), or a deformation of the structure (such as yttrium-barium-copper oxide).
2.Technical indicators:
Type | CT-1 | CT-2 | CT-3 | CT-4 |
Purity (wt%) | ≥99.5 | ≥99 | ≥99 | Adjustable |
MgO (wt%) | <0.05 | <0.10 | <1.00 | <3.00 |
Fe2O3 (wt%) | <0.05 | <0.10 | <0.50 | <3.00 |
K2O+Na2O (wt%) | <0.05 | <0.10 | <0.50 | Pb<0.01 |
Al2O3 (wt%) | <0.10 | <0.20 | <0.50 | <1.00 |
SiO2 (wt%) | <0.10 | <0.20 | <0.50 | <3.00 |
3. Packaging and storage:
Packaging specification:25kg/bag, also can be determined according to user's need.
During transportation, it is necessary to protect against sunlight, rain, and high temperatures.
The product should be stored in a cool, dark, ventilated place, away from sources of fire.
4.Safety protection:
Pay attention to labor protection when handling it and avoid contact with skin, eyes, etc.After contact, rinse immediately with plenty of water
Related keywords:
CALCIUM TITANATE
CAS No.12049-50-2
content is empty!