close
Choose Your Site
Global
Social Media
| Availability: | |
|---|---|
| Quantity: | |








56-84-8
bosschemical
56-84-8
L-Aspartic acid
CAS No.56-84-8
Alias:L-Aspartic acid L-ASPARTIC ACID; L-Aspartic acid Pharmaceutical grade 99%; L-Ascorbic acid, 98.5+%, LOW METALS CONTENT.
Molecular formula:C4H7NO4
Molecular weight:133.1
Structural formula:
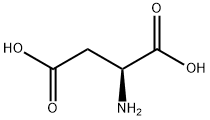
1. Performance and use:White crystal or crystalline powder, slightly acidic taste. Soluble in boiling water, slightly soluble in water at 25℃(0.5%), easily soluble in dilute acid and sodium hydroxide solution, insoluble in ethanol, ether, heated to 270℃ decomposition, isoelectric point 2Chemicalbook.77, its specific rotation is related to the solvent dissolved. In acid solution it is right-spin, in alkali solution it is left-spin, and in aqueous solution it is right-spin.
Used as an electrolyte supplement in amino acid infusions, inorganic ion supplements such as potassium and calcium, and fatigue recovery agents. Potassium and magnesium aspartate injection or oral solution is used for cardiac arrhythmia and premature beats caused by cardiac glycoside poisoning, tachycardia Chemicalbook tachycardia, hypokalemia, hypomagnesium, heart failure, myocardial infarction, angina pectoris, hepatitis, cirrhosis of the liver and other disorders. Low toxicity, the product is not diluted for injection, renal insufficiency and atrioventricular block with caution.
It can be used as ammonia detoxifier, liver function promoter, fatigue recovery agent and other medicines, food additives and additives for various cool drinks, also can be used as biochemical reagents, culture medium and intermediates for organic synthesis.
It can be used as synthetic sweetener, used in the treatment of heart disease, used as liver function promoter, ammonia detoxifier, fatigue eliminator and amino acid infusion components, etc.
Nutritional supplements, flavouring and aroma enhancers. It is added to various kinds of refreshing beverages. Used as ammonia detoxifier, liver function promoter, fatigue recovery agent.
Used in biochemical research, as fatigue recovery agent, ammonia detoxifier, clinical diagnostic agent.
Main neurotransmitter, causing rapid synaptic excitation.
2.Technical indicators
| ITEM | INSPECTION CRITERION | RESULT |
| Appearance | White crystals or crystalline powder | Conforms |
| Specific rotation[α]D 20 | +24.0°~+26.0° | +25.0° |
| Chloride(Cl) | ≤0.020% | <0.020% |
| Ammonium(NH4) | ≤0.02% | <0.02% |
| Sulfate(SO4) | ≤0.030% | <0.030% |
| Iron(Fe) | ≤10ppm | <10ppm |
| Heavy metals(Pb) | ≤10ppm | <10ppm |
| Chromatographic purity | Not more than 0.5% of any individual impurity is found; Not more than 2.0% of total impurities is found. | Any individual impurity<0.5%; Total impurities <2.0% |
| Loss on drying | ≤0.50% | 0.03% |
| Residue on ignition | ≤0.10% | 0.07% |
| Assay | 98.5%~101.5% | 99.3% |
3.Usage:
7.0% of total protein in food (including asparagine; FDA §172.320, 2000).FEMA (mg/kg): bakery products, meat products, bouillon, sauces, 250; nonalcoholic beverages 150.
4. Packaging and storage
Packaging specification: Packing specification: 25kg/bag, also can be determined according to user's need.
During transportation, it is necessary to protect against sunlight, rain, and high temperatures.
The product should be stored in a cool, dark, ventilated place, away from sources of fire.
5.Safety protection
Pay attention to labor protection when handling it and avoid contact with skin, eyes, etc.After contact, rinse immediately with plenty of water
Related keywords:
L-Aspartic acid
CAS 56-84-8
L-Aspartic acidUSP
L-Aspartic acid
CAS No.56-84-8
Alias:L-Aspartic acid L-ASPARTIC ACID; L-Aspartic acid Pharmaceutical grade 99%; L-Ascorbic acid, 98.5+%, LOW METALS CONTENT.
Molecular formula:C4H7NO4
Molecular weight:133.1
Structural formula:

1. Performance and use:White crystal or crystalline powder, slightly acidic taste. Soluble in boiling water, slightly soluble in water at 25℃(0.5%), easily soluble in dilute acid and sodium hydroxide solution, insoluble in ethanol, ether, heated to 270℃ decomposition, isoelectric point 2Chemicalbook.77, its specific rotation is related to the solvent dissolved. In acid solution it is right-spin, in alkali solution it is left-spin, and in aqueous solution it is right-spin.
Used as an electrolyte supplement in amino acid infusions, inorganic ion supplements such as potassium and calcium, and fatigue recovery agents. Potassium and magnesium aspartate injection or oral solution is used for cardiac arrhythmia and premature beats caused by cardiac glycoside poisoning, tachycardia Chemicalbook tachycardia, hypokalemia, hypomagnesium, heart failure, myocardial infarction, angina pectoris, hepatitis, cirrhosis of the liver and other disorders. Low toxicity, the product is not diluted for injection, renal insufficiency and atrioventricular block with caution.
It can be used as ammonia detoxifier, liver function promoter, fatigue recovery agent and other medicines, food additives and additives for various cool drinks, also can be used as biochemical reagents, culture medium and intermediates for organic synthesis.
It can be used as synthetic sweetener, used in the treatment of heart disease, used as liver function promoter, ammonia detoxifier, fatigue eliminator and amino acid infusion components, etc.
Nutritional supplements, flavouring and aroma enhancers. It is added to various kinds of refreshing beverages. Used as ammonia detoxifier, liver function promoter, fatigue recovery agent.
Used in biochemical research, as fatigue recovery agent, ammonia detoxifier, clinical diagnostic agent.
Main neurotransmitter, causing rapid synaptic excitation.
2.Technical indicators
| ITEM | INSPECTION CRITERION | RESULT |
| Appearance | White crystals or crystalline powder | Conforms |
| Specific rotation[α]D 20 | +24.0°~+26.0° | +25.0° |
| Chloride(Cl) | ≤0.020% | <0.020% |
| Ammonium(NH4) | ≤0.02% | <0.02% |
| Sulfate(SO4) | ≤0.030% | <0.030% |
| Iron(Fe) | ≤10ppm | <10ppm |
| Heavy metals(Pb) | ≤10ppm | <10ppm |
| Chromatographic purity | Not more than 0.5% of any individual impurity is found; Not more than 2.0% of total impurities is found. | Any individual impurity<0.5%; Total impurities <2.0% |
| Loss on drying | ≤0.50% | 0.03% |
| Residue on ignition | ≤0.10% | 0.07% |
| Assay | 98.5%~101.5% | 99.3% |
3.Usage:
7.0% of total protein in food (including asparagine; FDA §172.320, 2000).FEMA (mg/kg): bakery products, meat products, bouillon, sauces, 250; nonalcoholic beverages 150.
4. Packaging and storage
Packaging specification: Packing specification: 25kg/bag, also can be determined according to user's need.
During transportation, it is necessary to protect against sunlight, rain, and high temperatures.
The product should be stored in a cool, dark, ventilated place, away from sources of fire.
5.Safety protection
Pay attention to labor protection when handling it and avoid contact with skin, eyes, etc.After contact, rinse immediately with plenty of water
Related keywords:
L-Aspartic acid
CAS 56-84-8
L-Aspartic acidUSP
content is empty!